Electron orbitals are filled in the order
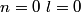 , , 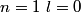 , , 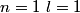 , , 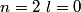 , , 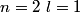 , , 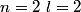 , etc until the fourth row of the periodic table (starting with potassium (Z = 19)). Sodium comes before this, so its orbitals are filled by first filling , etc until the fourth row of the periodic table (starting with potassium (Z = 19)). Sodium comes before this, so its orbitals are filled by first filling 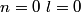 (2 electrons), then filling (2 electrons), then filling 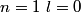 (2 more electrons), then filling (2 more electrons), then filling 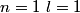 (6 more electrons), then filling the first electron in the (6 more electrons), then filling the first electron in the 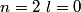 subshell. This electron configuration can be written as subshell. This electron configuration can be written as 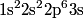 . Therefore, answer (C) is correct. . Therefore, answer (C) is correct. |